Good day........
This article is regrading the chronology of degradation studies
In pre-development, a limited scope (general) forced degradation study is typically performed for discovery candidate nominations
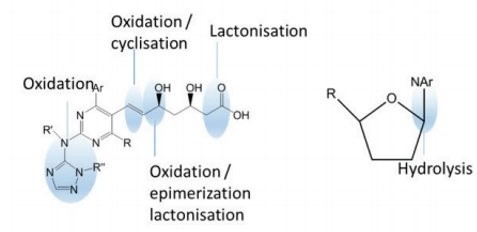
Additional and more focused molecular stability studies for discovery compounds transitioning into development may uncover known chemical conditions of instability. Degrdataion can be further understood with an evaluation of the chemical structure to determine likely high risk functional groups or 'soft spots" based on common known degradation pathways (e.g. Carboxylic acid or amide groups)
Molecular degradation predictions may also be evaluated using specialised softwares. Furthermore, DS/ excipient compatibility studies are used when scouting possible early formulations to control minimize the risk of degradation through formulation design
Additional controls may be put in place based on this knowledge (e.g. with use of appropriate packaging technologies such as the use of dexicants or antioxidants. Further knowledge may be obtained when performing stability assessment of DS, e.g., solid state from studies
Any significant degradation should be further investigated to determine degradant structure and mechanism of formation
Overall this foundation of knowledge provides a good starting point for a structural physico-chemical evaluation and subsequently a more tailored forced degradation protocol design for later stages of development
Thank you for the readers and following the FWQRC blogs